Treatment Information
Know your treatment options
Each cancer story is personal. Treatment should be personal too. Your healthcare team will recommend treatment options specifically for you. Options may include chemotherapy, targeted therapy, immunotherapy, radiation, or surgery.
There are several treatment options for people diagnosed with lung cancer. Physicians determine
treatment plans based on a variety of factors, including:
- The amount of primary tumors
- Stage of cancer
- Challenges to treatment
- Patient’s overall health
There are an various methods of primary treatment. Common primary treatment types include:
- Surgery which can be used to remover tumors or organs with stage 1, 2, and 3 lung cancer.
- Chemotherapy which can be used to kill cancer cells with powerful drugs.
- Radiation therapy which can be used as it utilizes high-energy x-rays to reach lung cancer.
- Chemoradiation which uses a comnination of radiation and chemotherapy.
- Targeted therapy which can be used to treat driver mutations of lung cancer by stopping specific ways the cancer cells live.
- Immunotherapy which utilizes the immune system to kill cancer cells.
To learn more, please visit the following links:
Non-Small Cell Lung Cancer – Early and Locally Advanced-English Version 2021
Non-Small Cell Lung Cancer – Metastatic-English Version 2021
Small Cell Lung Cancer-English Version 2022
Learn more about current treatment options from our treatment update at the 2021 Advocacy Summit by watching the video:

Comprehensive Biomarker Testing and Targeted Therapies
Comprehensive biomarker testing is a way for doctors to test the genetic makeup of a tumor and to help make the best decision possible about your treatment. The more that is known about the tumor determines how specific and targeted your treatment options can be.
About Comprehensive Biomarker Testing
Who should be tested?
How is testing done?
When should you get tested?
Where can you get tested?
Facts about DNA Mutations
Every living thing is made up of building blocks called cells. Inside of each cell is a set of instructions for how to do its job. These instructions are called DNA and mistakes in them are called mutations. Mutations can be inherited (rarely in lung cancer), occur due to carcinogens (cancer-causing chemicals such as those found in cigarette smoke) or through bad luck. Regardless, they can cause the cell to forget how to do its job and instead learn how to make copies of itself, spread and grow. A cell with such mutations is called a cancer cell.
In non-squamous (mostly adenocarcinoma) of the lung, we can identify the specific changes that happened early in the process of the once-healthy cell becoming cancerous. These events are called, “driver mutations.”
Some of these mutations have drugs that can target them. Targeted therapies mostly only work when matched to target. When tried in patients without the change that the targeted therapy actions, they tend to fail. But, when properly matched, these drugs tend to work better than chemotherapy. More specifically, their advantages are:
They work better: Response rate is arbitrarily defined as 30 percent shrinkage of cancer (it needed to be set somewhere to allow drugs to be compared to each other, but a patient with 29 percent shrinkage of cancer and a patient with 31 percent shrinkage of cancer really derive similar benefit, even if one is labeled as having “partial response” and the other as, “stable disease”). The response rate with targeted therapies is at least 66 percent and most of the remaining 1/3 of patients derive benefit less than 30 percent shrinkage. They also tend to control cancer longer than chemotherapy.
They’re less toxic: Targeted therapies are not side effect or risk free. But, they do tend to have a lot fewer side effects than chemotherapy. Side effects vary by agent, but rash and diarrhea are particularly common with these agents.
They’re more convenient: While most chemotherapy is IV, most targeted therapies are oral.
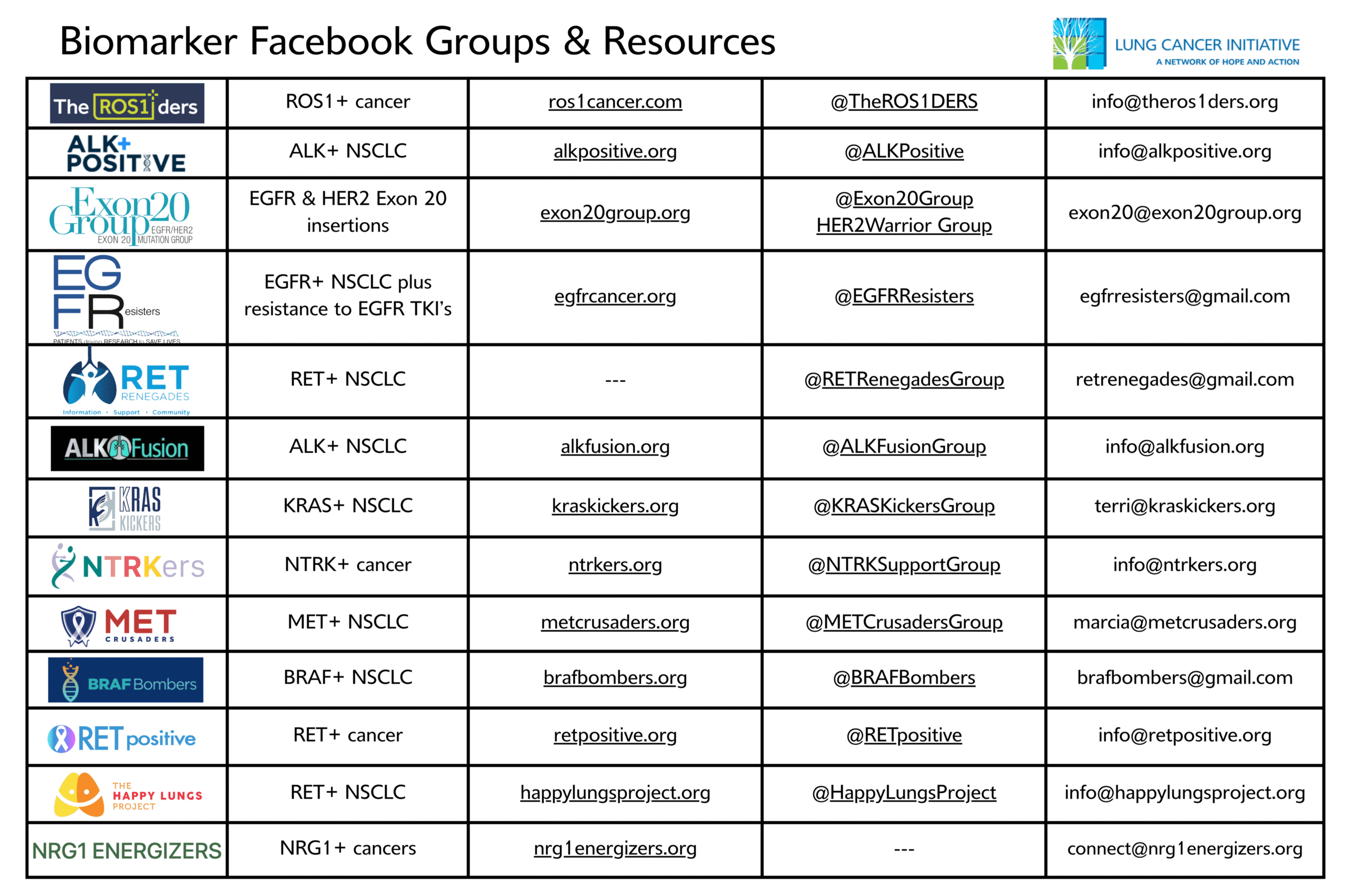
With that in mind, let’s talk about some important mutations:
EGFR:
ALK:
ROS1:
MET:
RET:
NTRK:
BRAF:
kRAS:
HER2:
Bridging the Comprehensive Biomarker Gaps
New biomarkers are being discovered at a rapid pace, unlocking new breakthrough treatments for patients and their families. Unfortunately, many patients still don’t get the biomarker testing they need, especially in North Carolina, where utilization of this important tool remains low.
That’s why the Lung Cancer Initiative is proud to join Lilly in a new campaign to expand access to comprehensive biomarker testing and give patients and providers clinically actionable information to inform their course of treatment.